Development of a biophramaceutical product is lengthy, complex, costly and risky. Without a proper drug development strategy, you are likely to fail.
With our expertise gained over more than 25 years, help you create and implement a complete clinical development plan that allows your company generate quality data, which meets regulatory and market access requirements, while optimizing timelines and resources used.
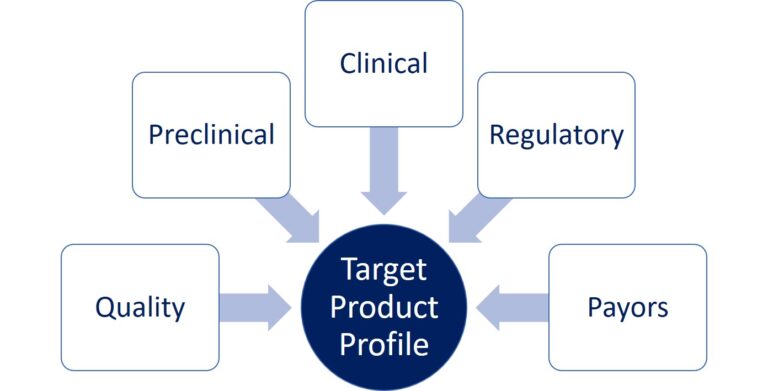
The development strategy that we will discuss and elaborate with you will include:
- Product risk assessment and market evaluation
- Precise pathway recommendation and countries selection
- Key studies required
- Efficient and effective studies designs
- Identification of clinical investigators,
- Strategic plans for regulatory interactions and submissions
Let’s discuss on how we can assist you to succeed with your clinical development strategy.
